Pentane (C₅H₁₂) is a hydrocarbon with five carbon atoms and twelve hydrogen atoms. It is part of the alkane family, which includes saturated hydrocarbons that follow the general formula CₙH₂ₙ₊₂. One fascinating aspect of organic chemistry is the concept of isomerism—when compounds have the same molecular formula but different structural arrangements.
In this article, we explore how many structural isomers can be drawn for pentane, why this number is fixed, what these isomers are called, and how their properties differ. This discussion is ideal for students, educators, and chemistry enthusiasts who want a deeper understanding of structural isomerism in simple alkanes.
What is a Structural Isomer?
A structural isomer (also called a constitutional isomer) is a compound that has:
- The same molecular formula as another compound
- A different structural arrangement of atoms
For example, both butane and isobutane have the formula C₄H₁₀, but they differ in how the carbon atoms are connected.
Isomers can have different:
- Boiling points
- Melting points
- Densities
- Reactivity
So, understanding how many structural isomers a compound can have is essential in organic chemistry.
The Molecular Formula of Pentane
Pentane has the molecular formula C₅H₁₂, which tells us:
- It contains 5 carbon atoms
- It contains 12 hydrogen atoms
- It is an alkane (saturated hydrocarbon, single bonds only)
The question is: How many unique ways can we arrange five carbon atoms to still satisfy this formula?
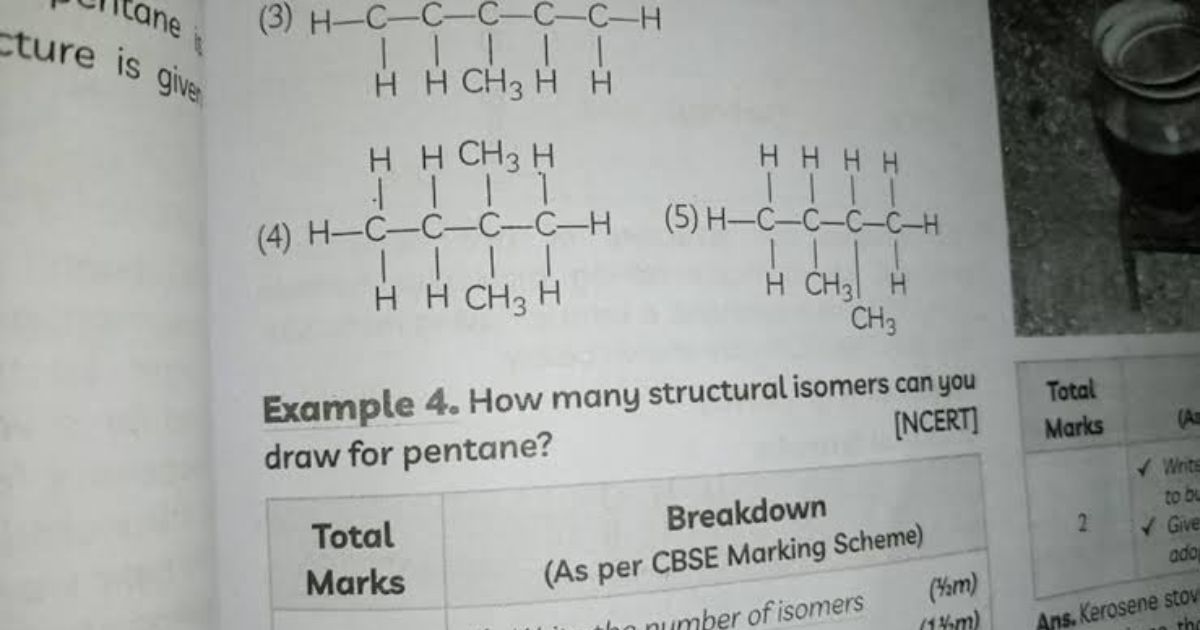
How Many Structural Isomers Can Be Drawn for Pentane?
The answer is: 3 structural isomers.
These three isomers are:
- n-Pentane
- Isopentane (or methylbutane)
- Neopentane (or dimethylpropane)
Each has the formula C₅H₁₂, but their carbon skeletons are arranged differently.
Let’s explore each one in detail.
1. n-Pentane (Normal Pentane)
Structure:
CH₃–CH₂–CH₂–CH₂–CH₃
Explanation:
- All five carbon atoms are connected in a straight chain.
- No branching occurs.
- This is the simplest form of pentane.
Properties:
- Boiling point: ~36°C
- Melting point: –130°C
- Density: 0.626 g/cm³
Notes:
- It is the most linear isomer.
- Due to less compactness, it has the highest boiling point among the three isomers.
2. Isopentane (Methylbutane)
Structure:
CH₃–CH(CH₃)–CH₂–CH₃
Explanation:
- A methyl group (CH₃) is attached to the second carbon of a butane backbone.
- This creates a branched chain.
Properties:
- Boiling point: ~28°C
- Melting point: –160°C
- Density: 0.620 g/cm³
Notes:
- More branched than n-pentane.
- Boiling point is slightly lower due to the decreased surface area.
3. Neopentane (Dimethylpropane)
Structure:
C(CH₃)₄
(A central carbon atom bonded to four methyl groups)
Explanation:
- The central carbon atom is bonded to four CH₃ groups.
- This is the most compact and highly branched isomer of pentane.
Properties:
- Boiling point: ~9.5°C
- Melting point: –16.6°C
- Density: 0.620 g/cm³
Notes:
- Highly symmetrical structure.
- Has the lowest boiling point due to minimal surface area for van der Waals interactions.
Summary Table of Pentane Isomers
| Isomer | Structure Type | Boiling Point | Melting Point | Branching Level |
|---|---|---|---|---|
| n-Pentane | Straight chain | ~36°C | ~–130°C | None |
| Isopentane | One branch (methyl) | ~28°C | ~–160°C | Moderate |
| Neopentane | Four methyl groups | ~9.5°C | ~–16.6°C | High |
Why Only 3 Structural Isomers for Pentane?
The limitation comes from:
- The valency of carbon (each carbon can form 4 bonds)
- The need to avoid repeating or identical structures (isomers must be unique)
If you try to draw more than three structures for C₅H₁₂, you’ll find:
- Either you’re repeating an existing one with a rotated or flipped shape
- Or you’re violating the valency rule
Therefore, only three valid structural isomers exist for pentane.
Are There Other Types of Isomers for Pentane?
For simple alkanes like pentane, we mainly consider structural isomers based on the carbon skeleton. However, in larger or more complex compounds, you can encounter:
- Positional isomers – Same skeleton, different position of functional groups
- Functional isomers – Different functional groups with the same formula
- Stereoisomers – Same connectivity, different spatial orientation
Pentane, being a saturated hydrocarbon without functional groups or double bonds, does not have these additional isomer types.
Applications and Importance of Pentane Isomers
Each isomer of pentane has different industrial uses and properties due to their molecular structure.
n-Pentane:
- Used in refrigerants, blowing agents, and solvents
- More volatile and flammable
Isopentane:
- Commonly used in geothermal power stations
- Used in fuel research
Neopentane:
- Used as a reference fuel
- Sometimes used in aerosol propellants
How to Identify Structural Isomers
Here are tips to draw and identify isomers of pentane (or any hydrocarbon):
- Start with the longest chain possible – In this case, 5 carbon atoms.
- Introduce branches – Try placing one or more methyl (CH₃) groups in different positions.
- Avoid duplication – Structures that look different might actually be the same upon rotation or reflection.
- Ensure correct valency – Carbon should have exactly four bonds.
- Verify molecular formula – Make sure the total atoms match C₅H₁₂.
Practicing with molecular models or drawing software can help in visualizing isomers better.
Quiz Yourself: Can You Spot the Difference?
Try drawing these three isomers yourself and label each one. Then try rotating or flipping them to see if they are unique.
Bonus Question:
What happens if you try drawing a 4-carbon chain with two methyl groups?
Answer: You will end up redrawing neopentane.
Conclusion: How Many Structural Isomers Can Be Drawn for Pentane
To summarize, only three structural isomers can be drawn for pentane (C₅H₁₂):
- n-Pentane – straight chain
- Isopentane (methylbutane) – one methyl branch
- Neopentane (dimethylpropane) – fully branched
These isomers highlight the concept of structural variation in organic molecules, and even with the same formula, their boiling points, melting points, and uses vary significantly.
Understanding isomers is a foundational skill in organic chemistry and plays a critical role in drug development, materials science, and fuel research.
So next time you see a simple hydrocarbon formula, try to imagine how many unique ways its atoms can be arranged—because in chemistry, structure truly defines function.